Abstract
The efficacy and long-term effectiveness of hydroxycarbamide/hydroxyurea (HU) in the prevention of painful crises and in the decrease of mortality and morbidity in sickle cell disease (SCD) patients have been established (Charache et al. 1992; Steinberg et al 2010, Voskaridou et al 2010).
From January 2009 to March 2019, the non-interventional prospective cohort study ESCORT-HU was conducted to evaluate the use of HU in real-life conditions and to collect information on the long-term safety of HU when used in current practice for the prevention or treatment of symptomatic complications in patients with sickle cell disease (SCD) (Montalembert et al 2021). A total of 1906 patients, 55% of adults, were enrolled in this study in 62 centres (Germany, Greece, Italy and France). The mean exposure to HU in this cohort was 30 months, for a cumulative exposure of 7310 patient-per year. The main objectives of ESCORT-HU have been fulfilled as regards the collection of data on the most common concerns associated with the use of HU in SCD patients: myelosuppression, child growth, concomitant administration of live vaccines, safety in population with renal and hepatic impairment and frequency of SCD events (painful crises, acute chest syndrome, stroke, acute splenic sequestration, infections, blood transfusions and hospitalizations) (Montalembert et al 2021).
In order to better identify potential long-term risks and specific concerns of HU therapy, ESCORT-HU extension is being implemented in Europe with the goal of 2500 patients enrolled. Main risks targeted by the study are leg ulcers, one of the most limiting factors to continue a treatment with HU. Patients will be recruited over a 5-year period. In addition to patients already involved in the first ESCORT-HU study, new at-risk patients might be added such as
patients with a history of HU exposure of at least 5 years, to allow a follow-up of patients treated with long-term HU to fully estimate the incidence of potential risk of malignancies
prepubescent children aged more than 10 years for girls and more than 13 years for boys in order to document impact or not of HU on puberty and realisation of cryopreservation,
patients with a history of leg ulcers, to search for discriminating criteria between leg ulcer caused by the disease and HU-induced leg ulcer,
pregnant women without interruption of HU 3 months before the beginning of the pregnancy and males treated with HU whose partner is pregnant and without discontinuation of HU 3 months before the beginning of the pregnancy. To date, there is a limited number of pregnancies exposed to HU with documented outcome. Despite no adverse effects on pregnancy or on the health of the foetus/new-born have been registered, an increase of the number of pregnancies with documented outcomes will allow for meaningful assessment of foetal outcome following exposure to HU during pregnancy,
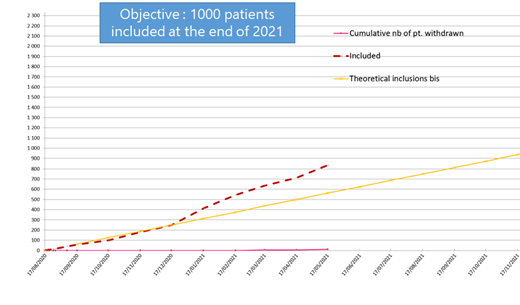
10 months after the beginning of ESCORT-HU extension, 818 patients have been enrolled in 70 investigational sites in 4 countries (Greece, Italy, Germany and France) (see Graph below). A first steering committee was hold in June 2021. Its conclusions were that distribution of patients (genotype, sex, age) was consistent with the first study and the number of events reported until now was coherent with what was expected per year, with no occurrence of major concern.
This extension study involves most of competence centres which manage SCD patients. SC patients have become increasingly well cared for in recent decades and have seen their life expectancy increase. HU treatment is now a chronic treatment possibly for life in many patients with SCD, better its knowledge of its efficiency and tolerance, better patient adherence to treatment will be.
de Montalembert: Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vertex: Consultancy. Voskaridou: ADDMEDICA: Consultancy, Research Funding; BMS: Consultancy, Research Funding; GENESIS: Consultancy, Research Funding; NOVARTIS: Research Funding; PROTAGONIST: Research Funding; IMARA: Research Funding. Oevermann: NOVARTIS: Consultancy; GBT: Consultancy. Joseph: bluebird bio: Consultancy. Colombatti: BlueBirdBio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees. Bartolucci: INNOVHEM: Other: Co-founder; Jazz Pharma: Other: Lecture fees; AGIOS: Consultancy; Addmedica: Consultancy, Other: Lecture fees, Research Funding; Fabre Foundation: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Lecture fees, Steering committee, Research Funding; Bluebird: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy; GBT: Consultancy; Emmaus: Consultancy; Hemanext: Consultancy. Brousse: BLUEBIRDBIO: Consultancy; ADDMEDICA: Consultancy. Galactéros: Addmedica: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal